Abstract

Introduction: Myelofibrosis (MF), whether primary (PMF) or secondary (SMF) to polycythemia vera or essential thrombocytemia, is characterized by a complex and partially undeciphered molecular architecture. Besides mutations in driver genes (JAK2, CALR, MPL), somatic mutations in selected myeloid-associated genes have been shown to impact prognosis of MF patients (pts). Among these, ASXL1 mutations (ASXL1MTs) are associated with poor outcomes in myeloid malignancies including PMF, where they are included in the category of "high molecular risk" (HMR) mutations along with EZH2MTs, IDH1/2MTs, and SRSF2MTs (Vannucchi AM, Leukemia 2013). However, a recent study (Luque Paz D, Blood Adv 2021) questioned the value of ASXL1MTs in MF. The current study aimed at further characterizing the prognostic role of ASXL1MTs in MF.
Methods: After IRB approval, pts with WHO-defined MF were included in the study. Mutational analysis by targeted NGS was performed as previously described (Guglielmelli P, JCO 2017). All deposited variants were manually curated to assess pathogenicity. In this study, we also used the molecular model proposed by Luque Paz et al. that identifies 4 genetic groups: TP53MT; High-risk (≥1 mutation in EZH2, CBL, U2AF1, SRSF2, IDH1/2); ASXL1MT-only; and "Others".
Results: A total of 525 pts were included in the study, including 331 (63%) PMF and 194 (37%) SMF. Median age at diagnosis was 89 (18-90) years, 314 (60%) were male. The median follow-up time was 80 (98% CI, 68-90) months. Overall, 324 (62%) pts were JAK2MT, 126 (24%) CALRMT, 24 (5%) MPLMT, 40 (8%) triple negative (TN), and 11 (2%) double mutated. Among non-driver genes, ASXL1MTs were found in 158 (30%) pts, EZH2MTs in 45 (9%), SRSF2MTs in 37 (7%), NRASMTs in 30 (6%) U2AF1MTs in 27 (5%), TP53MTs and CBLMTs in 25 (5%) each, IDH1/2 MTs in 18 (3%), and KRAS MTs in 15 (3%). Pts in the HMR category were 125 (38%) in PMF and 63 (32%) in SMF.
According to the above model, distribution of pts was as follows: TP53MT n=25 (5%), High-risk n=137 (26%), ASXL1MT-only n=64 (12%), and Others n=299 (57%). Pts in the TP53MT and ASXL1MT-only groups were more likely to be diagnosed with SMF compared to pts in the High-risk and Others groups (44% and 48% vs 28% and 38%, respectively). In addition, the High-risk group was enriched in TN pts (16%), while CALRMTs were more common in the ASXL1MT-only and Others compared to the TP53MT and High-risk groups (25% and 27% vs 12% and 18%, respectively).
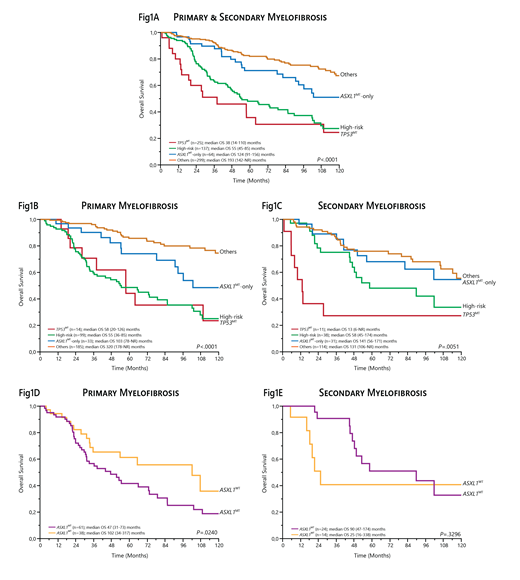
In univariate analysis, the TP53MT and High-risk groups were associated with the worst overall survival (OS), with median values of 38 (14-110) and 55 (45-85) months (P=.0039), respectively (Fig 1A). Albeit remarkably better, the OS of pts in the ASXL1MT-only group was inferior compared to pts in the Others group (median 124 [91-156] vs 193 [142-NR] months; P=.0118) (Fig 1A).
We then analyzed separately PMF and SMF cohorts. In the former, the TP53MT and High-risk groups remained associated with the worst OS (median 58 [20-126] vs 55 [36-85] months), although with no significant difference, likely due to the low frequency (4%) of TP53MTs mutations in PMF (Fig 1B). Concurrently, the negative prognostic impact of the ASXL1MT-only group was confirmed in comparison to the Others group (median 103 [78-NR] vs 320 [178-NR] months; P=.0170). In pts with SMF, while the TP53MT group (6%) had by far the worst OS (median 13 [6-NR] months), the OS of the ASXL1MT-only group (median 141 [56-171] months) was comparable to that of the Others group (median 131 [106-NR] months; P=.5188) and not different from the High-risk group (median 58 [45-174] months; P=.3606) (Fig 1C). In a further analysis including only pts in the High-risk group, ASXL1MTs were found in 62% and 63% of patients with PMF and SMF, respectively. In survival analysis, the presence of ASXL1MTs was associated with an increased risk of death only in PMF (median OS 47 [31-73] vs 102 [34-317] months; P=.0240), unlike in SMF (median OS 90 [47-174] vs 25 [16-338] months; P=.3296) (Fig 1D-E).
Conclusion: In the current study, we critically re-addressed the prognostic impact of ASXL1MTs by applying a genetic model recently developed by Luque Paz et al. to our cohort of molecularly annotated, WHO-defined MF pts. Overall, our results confirm that ASXL1MTs -even in the absence of other co-occurring high-risk mutations- harbor a negative prognostic impact mainly in PMF. These findings also reinforce the idea that PMF and SMF represent two different biological entities.
Vannucchi: Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal